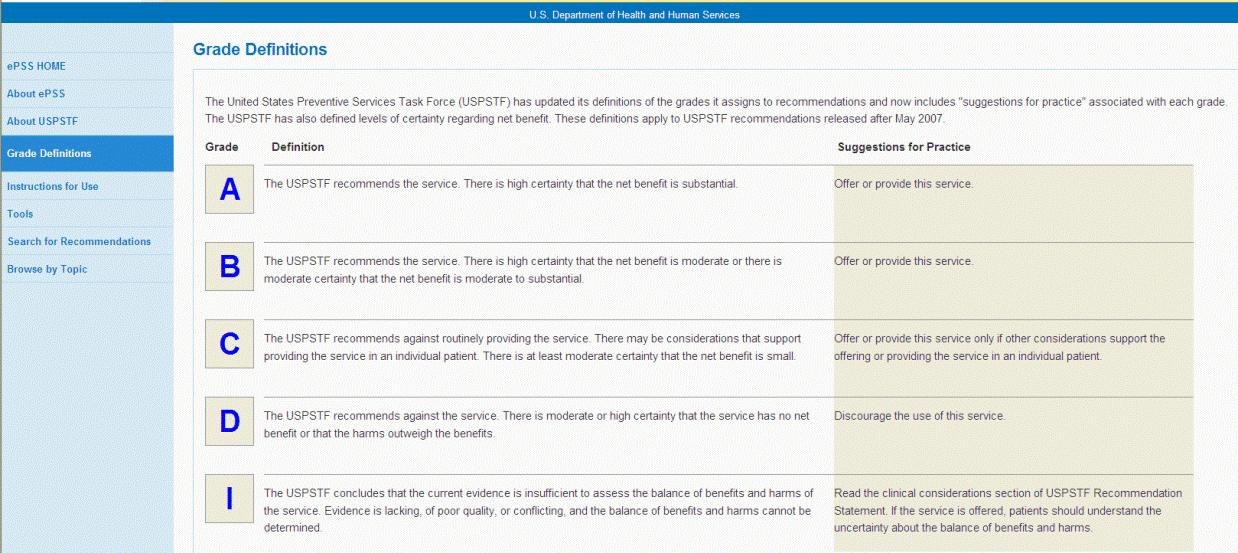
1. Can the results be applied to my
patients?
2. Were all clinically relevant outcomes
considered?
3. Are the benefits worth the harms and
costs?
REMEMBER: Evidence makes an important
contribution to good clinical decision making but evidence,
though
necessary, is not always sufficient. A clinician must also deal with a patient's
values and preferences.
In addition to clinical expertise, a good
clinician must demonstrate compassion, sensitive listening skills,
and broad perspectives from the humanities and social
sciences. These attributes allow for an understanding
of patients'
illnesses in the context of their experience, personalities,
and cultures.
_______________________________________________________________________________
Clinical Epidemiology Glossary
Absolute risk: The observed or calculated
probability of an event in the population under study.
Absolute risk difference: the difference in the
risk for disease or death between an exposed population
and an unexposed population.
Absolute risk reduction (ARR):
the difference in the absolute risk (rates of adverse
events) between study and control populations.
Adjustment: A summarizing procedure for a
statistical measure in which the effects of differences in
composition of the populations being
compared have been
minimized by statistical methods.
Association: Statistical dependence between two or
more events, characteristics, or other variables. An
association may be fortuitous or may
be produced by various
other circumstances; the presence of an association does not
necessarily imply a causal relationship.
Bias (Syn: systematic error):
Deviation of results or inferences from the truth, or
processes leading to such deviation. See also
Referral Bias,Selection
Bias.
Blind(ed) study (Syn: masked
study): A study in which observer(s) and/or subjects are
kept ignorant of the group to which the subjects are
assigned, as
in an experimental study, or of the population
from which the subjects come, as in a nonexperimental or
observational study. Where both observer and
subjects are
kept ignorant, the study is termed a double-blind
study. If the statistical analysis is also done in ignorance
of the group to which subjects
belong, the study is
sometimes described as triple blind. The purpose of
"blinding" is to eliminate sources of bias.
Case-series: Report of a number of cases of
disease.
Case-control study:
Retrospective comparison of exposures of persons with
disease (cases) with those of persons without the disease
(controls)
(see Retrospective
study).
Causality: The relating of causes to the effects
they produce. Most of epidemiology concerns causality and
several types of causes can be distinguished.
It must be
emphasized, however, that epidemiological evidence by itself
is insufficient to establish causality, although it can
provide powerful circumstantial evidence.
Co-interventions: Interventions
other than the treatment under study that are applied
differently to the treatment and control groups.
Cointervention is a
serious problem when double blinding is
absent or when the use of very effective non-study
treatments is permitted.
Cohort study: Follow-up of
exposed and non-exposed defined groups, with a comparison of
disease rates during the time covered.
Comparison group: Any group to which the index
group is compared. Usually synonymous with control group.
Co-morbidity: Coexistence of a
disease or diseases in a study participant in addition to
the index condition that is the subject of study.
Confidence interval (CI): The
range of numerical values in which we can be confident (to a
computed probability, such as 90 or 95%) that the population
value being estimated will be found. Confidence intervals
indicate the strength of evidence; where confidence
intervals are wide, they indicate less precise
estimates of
effect. The larger the trial's sample size, the larger the
number of outcome events and the greater becomes the
confidence that the true relative
risk reduction is close to
the value stated. Thus the confidence intervals narrow and
"precision" is increased. In a "positive finding" study the
lower boundary
of the confidence interval, or lower
confidence limit, should still remain important or
clinically significant if the results are to be accepted. In
a "negative finding"
study, the upper boundary of the
confidence interval should not be clinically significant if
you are to confidently accept this result.
Confounding variable, Confounder:
A variable that can cause or prevent the outcome of
interest, is not an intermediate variable, and is associated
with the
factor under investigation. A confounding variable
may be due chance or bias. Unless it is possible to adjust
for confounding variables, their effects cannot be
distinguished from those of factor(s) being studied.
Control event rate(CER): The
percentage of the control/nonexposed group who experienced
outcome in question.
Dose-response relationship: A
relationship in which change in amount, intensity, or
duration of exposure is associated with a change-either an
increase or
decrease-in risk of a specified outcome.
Determinant: Any definable factor that effects a
change in a health condition or other characteristic.
Effectiveness: a measure
of the benefit resulting from an intervention for a given
health problem under usual conditions of clinical care for a
particular group;
this form of evaluation considers both the
efficacy of an intervention and
its acceptance by those to whom it is offered, answering the
question, "Does the
practice do more good than harm to
people to whom it is offered?" See
Intention to treat.
Efficacy: a measure of the
benefit resulting from an intervention for a given health
problem under the ideal conditions of an investigation; it
answers the question,
"Does the practice do more good than
harm to people who fully comply with the recommendations?"
Exclusion Criteria: Conditions which preclude
entrance of candidates into an investigation even if they
meet the inclusion criteria.
Experimental event rate(EER):
The percentage of intervention/exposed group who experienced
outcome in question.
Follow-up: Observation over a
period of time of an individual, group, or initially defined
population whose relevant characteristics have been assessed
in order
to observe changes in health status or
health-related variables.
Gold standard: A method, procedure, or measurement
that is widely accepted as being the best available.
Incidence: The number of new cases of illness
commencing, or of persons falling ill, during a specified
time period in a given population. See also
Prevalence.
Intention to treat analysis: A
method for data analysis in a randomized clinical trial in
which individual outcomes are analyzed according to the
group to which
they have been randomized, even if they never
received the treatment they were assigned. By simulating
practical experience it provides a better measure of
effectiveness (versus
efficacy).
Interviewer bias: Systematic
error due to interviewer's subconscious or conscious
gathering of selective data.
Lead-time bias: If prognosis study patients are
not all enrolled at similar, well-defined points in the
course of their disease, differences in outcome over time
may
merely reflect differences in duration of illness.
Likelihood ratio: Ratio of the
probability that a given diagnostic test result will be
expected for a patient with the target disorder rather than
for a patient without the disorder.
Number Needed to Treat (NNT):
the number of patients who must be exposed to an
intervention before the clinical outcome of interest
occurred; for example, the
number of patients needed to
treat to prevent one adverse outcome.
Odds: a proportion in which the numerator contains
the number of times an event occurs and the denominator
includes the number of times the event does not occur.
Odds Ratio (Syn: cross-product
ratio, relative odds): a measure of the degree of
association; for example, the odds of exposure among the
cases compared with the
odds of exposure among the controls.
Precision: The range in which the best estimates
of a true value approximate the true value. See
Confidence interval.
Predictive value: In
screening and diagnostic tests, the probability that a
person with a positive test is a true positive (i.e., does
have the disease), or that a person
with a negative test
truly does not have the disease. The predictive value of a
screening test is determined by the
sensitivity and specificity
of the test, and by the
prevalence of the condition for
which the test is used.
Prevalence: the proportion of
persons with a particular disease within a given population
at a given time.
Prognosis: the possible
outcomes of a disease or condition and the likelihood that
each one will occur.
Prognostic factor:
Demographic, disease-specific, or co-morbid characteristics
associated strongly enough with a condition's outcomes to
predict accurately the
eventual development of those
outcomes. Compare with risk factors.
Neither prognostic or risk factors necessarily imply a cause
and effect relationship.
Prospective study: Study design where one or more
groups (cohorts) of individuals who have not yet had
the outcome event in question are monitored for the
number
of such events which occur over time.
Randomized controlled trial:
Study design where treatments, interventions, or enrollment
into different study groups are assigned by random
allocation rather
than by conscious decisions of clinicians
or patients. If the sample size is large enough, this study
design avoids problems of bias and confounding variables by
assuring that both known and unknown determinants of outcome
are evenly distributed between treatment and control groups.
Recall bias: Systematic error
due to the differences in accuracy or completeness of recall
to memory of past events or experiences.
Referral filter bias: The
sequence of referrals that may lead patients from primary to
tertiary centres raises the proportion of more severe or
unusual cases,
thus increasing the likelihood of adverse or
unfavorable outcomes.
Relative risk (RR): the ratio of
the probability of developing, in a specified period of
time, an outcome among those receiving the treatment of
interest or
exposed to a risk factor, compared with the
probability of developing the outcome if the risk factor or
intervention is not present.
Relative risk reduction (RRR):
the extent to which a treatment reduces a risk, in
comparison with patients not receiving the treatment of
interest.
Reliability
(Repeatability, Reproducibility): the results of a test or
measure are identical or closely similar each time it is
conducted.
Retrospective study: study
design in which cases where individuals who had an outcome
event in question are collected and analyzed after the
outcomes
have occurred (see also
Case-control study).
Risk factor: patient
characteristics or factors associated with an increased
probability of developing a condition or disease in the
first place. Compare with
prognostic
factors. Neither risk or prognostic factors
necessarily imply a cause and effect relationship.
Selection Bias: a bias in
assignment or a confounding variable
that arises from study design rather than by chance. These
can occur when the study
and control groups are chosen so
that they differ from each other by one or more factors that
may affect the outcome of the study.
Sensitivity (of a diagnostic
test): the proportion of truly diseased persons, as measured
by the gold standard, who are identified as diseased by the
test under study.
Specificity (of a diagnostic
test): the proportion of truly nondiseased persons, as
measured by the gold standard, who are so identified by the
diagnostic test under study.
Stratification: division into groups.
Stratification may also refer to a process to control for
differences in confounding variables, by making separate
estimates for
groups of individuals who have the same values
for the confounding variable.
Strength of Inference: the likelihood that an
observed difference between groups within a study represents
a real difference rather than mere chance or
the influence
of confounding factors, based on both p values and
confidence intervals. Strength of inference is weakened by
various forms of bias and by small sample sizes.
Survival curve: A graph of
the number of events occurring over time or the chance of
being free of these events over time. The events must be
discrete and the
time at which they occur must be precisely
known. In most clinical situations, the chance of an outcome
changes with time. In most survival curves the earlier
follow-up periods usually include results from more patients
than the later periods and are therefore more precise.
Validity: the extent to which a
variable or intervention measures what it is supposed to
measure or accomplishes what it is supposed to accomplish.
The internal validity of a study refers to the
integrity of the experimental design.
The external validity of a study refers to the
appropriateness by which its results can be applied to
non-study patients or populations.
 Prevention, Screening & Health Maintenance
Prevention, Screening & Health Maintenance